9 Unlock Empirical Formula How To Find - You then divide by the smallest number of moles and construct the empirical formula from the. It explains how to find the empirical formula of a hydrated ionic compound.my website:

Empirical formula how to find
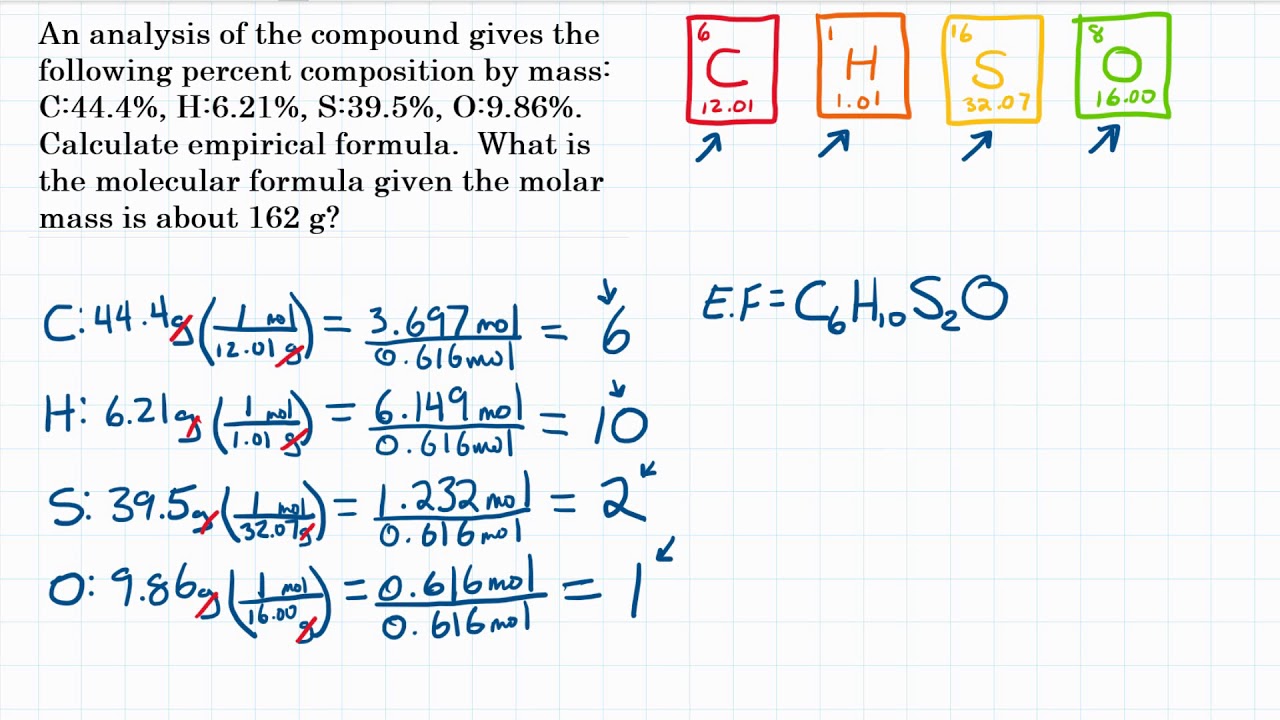
9 Expert Empirical Formula How To Find. The molecular formula of ribose is c 5 h 10 o 5, which can be reduced to the empirical formula ch 2 o. Determine the masses of each component in the compound. The empirical formula shows the simplest ratio of elements in a compound also called simple formulas. Empirical formula how to find
The steps are:1) write the atoms involved in the calculation.2) write the mas. So, the simplest formula of the compound is ch2o. To find the ratio between the molecular formula and the empirical formula. Empirical formula how to find
If it is 2, the i will not divluge the answer to you, megan, since This program determines both empirical and molecular formulas. The molecular formula of a compound may be the empirical formula, or it may be a multiple of the empirical formula. Empirical formula how to find
C=40%, h=6.67%, o=53.3%) of the compound. Molecular formulas show all atoms of each element in a molecule. It contains 2 moles of hydrogen for every mole of carbon and oxygen. Empirical formula how to find
Know about molecular and empirical formulas here. Determine the number of moles by dividing the grams by the atomic mass. This chemistry video tutorial focuses on hydrates. Empirical formula how to find
Convert numbers to whole numbers by multiplying by the smallest number that can make the decimals. This chemistry video tutorial explains how to find the empirical formula given the mass in grams or from the percent composition of each element in a compoun. That would give a ratio of 1.5:1 in the previous example, but to get to whole numbers you need to multiply by. Empirical formula how to find
To find the molecular formula, you must be given the actual molar mass of the compound. The empirical formula is the simplest ratio of the number of atoms involved in the compound's formation. In the study of a chemical system, we need to represent elements and compounds very frequently. Empirical formula how to find
Formula to calculate empirical formula. Also, our online empirical formula calculator considers these equations for finding the simplest positive integer ratio of atoms present in a compound (chemistry). Visit byju's to learn more about it. Empirical formula how to find
Basically, the mass of the empirical formula can be computed by dividing the molar mass of the compound by it. This video goes into detailed steps on how to find the empirical formula of a compound. The empirical formula of a compound is the simplest whole number ratio of each type of atom in a compound. Empirical formula how to find
It can be the same as the compound's molecular formula, but not always.an empirical formula can be calculated from information about the mass of each element in a compound or from the percentage composition. 50% can be entered as.50 or 50%.) Divide the number of moles of each element by the smallest number of moles. Empirical formula how to find
The empirical formula of a substance can be calculated from the experimentally determined percent composition, the percentage of each element present in a how do you find the percent concentration of a solution? To find the empirical formula from at% you need to divide by the lowest value to reduce that to 1 and preserve the ratio. Empirical formula of a hydrocarbon containing 80% carbon and 20% h | filo. Empirical formula how to find
Formula for empirical probability in empirical probability, we have a formula we use to calculate probabilities: We calculate empirical probability by dividing the number of times an event occurred during our experiment or observation by the total number of trials or observations. Percentages can be entered as decimals or percentages (i.e. Empirical formula how to find
Glucose has a molecular formula of c 6 h 12 o 6. Enter an optional molar mass to find the molecular formula. For example, if it is one, the empirical formula is equal to the molecular formula. Empirical formula how to find
Multiply every atom (subscripts) by this Percent composition is usually used to find the empirical formula. The empirical formula for glucose is ch 2 o. Empirical formula how to find
To calculate the empirical formula, enter the composition (e.g. You divide the percentage of each atom by its’ molar mass and calculate the number of moles of each atom. Of moles simple ratio c = 80% 80/12 = 6.66 1 h = 20% 20/1 = Empirical formula how to find









